Pharma logistics for
the post-Covid era

- The Covid-19 pandemic is now largely behind us, thanks to the remarkable pace at which vaccines were developed, a process that typically spans years but was accomplished in a matter of months. Within this success lies the hidden triumph of pharmaceutical manufacturing and logistics.
- The deployment of Covid vaccines was the most extensive vaccination campaign in history, and has been referred to as the world's largest ever product launch. Some 13bn doses were administered globally, with 70 per cent of the world's population receiving at least one dose. What makes the feat even more astounding is that the new class of mRNA vaccines requires refrigeration, some as low as minus 70 degrees Celsius, throughout production, storage and distribution.
- "A pandemic means there is disease everywhere, which implies drugs must also be delivered everywhere," says Satoshi Otsuji, executive officer of NIPPON EXPRESS HOLDINGS, INC. "And in this case, vaccines had to be delivered under temperature-controlled conditions. The scale and speed of all this was unprecedented for us, as it was for all logistics providers, but I think as an industry we were able to meet this challenge through nimble thinking and flexible solutions. "
- Many global logistics providers, including the Nippon Express Group (NX Group), chartered grounded passenger flights to deliver vaccines, using cold-storage containers and boxes with masses of dry ice to keep vital deliveries continuing during the pandemic.
- It was also fortuitous that the Japan-based group, the world's eighth-largest logistic provider by gross logistics revenue and freight forwarding volumes, and Asia's largest, had committed to expanding its pharma business a few years before the outbreak. The NX Group entered the pharmaceutical logistics industry in 2018, anticipating the growing demand for the domestic pharmaceutical industry to comply with good distribution practice (GDP) guidelines.
- These GDP standards, already legally mandated in the EU, have triggered a sea change in how pharmaceuticals are manufactured and delivered. The standards require distributors to guarantee the quality and integrity of pharmaceuticals and their active ingredients throughout the supply chain from factory to patient. Complying with GDP combats the unacceptable practices of counterfeit pharmaceuticals and theft. It also ensures the integrity of certain pharmaceuticals, such as biological drugs and vaccines, which are highly sensitive to temperature changes.
-

- Otsuji explains that, by the time the pandemic struck, the NX Group had already established four major GDP-certified warehouses in Japan while busy building a global network of warehouses, distribution centres and dedicated vehicles for pharma. It was also working on a digital platform to ensure end-to-end visibility of conditions -- from temperature, humidity, shocks, to light -- during the transport of sensitive items including pharmaceuticals and semiconductors.
- "Covid really tested and trained our cold-chain capabilities," says Otsuji.
- The future of pharma logistics
- This experience will be invaluable for post-Covid pharma logistics.
- Otsuji expects a continued industry trend towards preventative medicine and highly specialised pharmaceuticals, such as mRNA therapeutics, which require sub-zero refrigeration for production and distribution. This will involve shorter lead times for delivery, along with greater need for real-time visibility of shipments employing new IoT devices, AI-based solutions and platforms being developed by the industry.
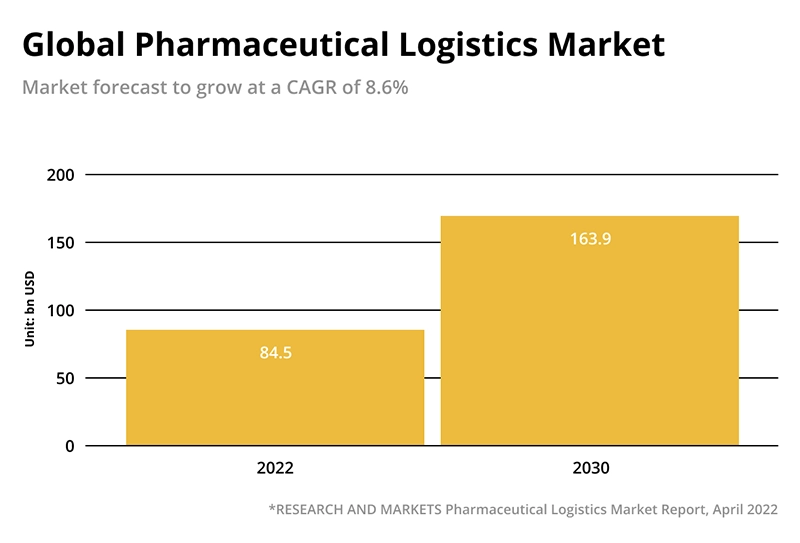
- However, not all players will be capable of meeting the highly complex logistics requirements essential for establishing reliable cold chains that comply with the ever-evolving and increasingly rigorous GDP and local regulations. For those who can successfully navigate this challenge, business should be good. According to a recent industry report, the global pharmaceutical logistics market, valued at $84.5bn in 2022, is projected to grow by a CAGR of 8.6 per cent up to 2030.
-
- By expanding its network of assets, know-how and a reputation for meticulous quality, the NX Group is capturing a larger share of this pie. "Revenues for pharma logistics reached ¥61.3bn ($400m) for FY2022, with sales from outside of Japan already surpassing the company's mid-term target set four years ago."
Among its five focus industries, its pharmaceutical and medical sectors grew particularly rapidly. - As of April 2023, the company has developed a network of 35 GDP-certified stations in 25 countries. By acquiring MD Logistics in 2020, a leading logistics provider for life sciences and pharmaceuticals in the US, the NX Group has raised its presence in the world's largest pharma consumer market. Earlier this year, it opened a dedicated warehouse in North Carolina, home to a booming biotechnology cluster.
- "Few logistics providers have a strong base in the world's largest and third-largest pharma markets by consumption, the US and Japan, and offer end-to-end forwarding services connecting these two markets," says Otsuji.
- The firm hopes to leverage its geographic reach by expanding in the Indian market, where it already conducts strong business in forwarding generic drugs to Japan, the US and Europe. It is considering organic and non-organic growth strategies for the European market, a key logistics market for the pharmaceutical industry.
- Towards a more sustainable and reliable pharma cold chain
- Preparing for future trends in pharma, including potential disruptions like another pandemic, will require evolving existing technologies and networks to achieve even more resilient supply chains.
Advances in real-time monitoring, such as those made by NX's partners, will be essential to enable more cost-effective and sustainable logistics solutions, particularly in areas such as vaccines. - Reducing the environmental footprint of pharma logistics also remains a challenge. Storing and transporting items at ultra-low temperatures is energy intensive. Even for cold chain transport using so-called "passive" packaging, it is necessary to choose reusable materials. Furthermore, IoT devices (loggers) used to provide real-time monitoring of temperature during transport also need to be reusable, rather than single use. Otsuji says that the NX Group is working on reusing these materials to enhance the sustainability of cold chains.
- But ultimately, where human lives are directly at stake, the sine qua non is reliability. Otsuji emphasises how the NX Group prides itself on its meticulous and uncompromising quality control, down to the smallest detail, throughout its end-to-end logistics operations. With the recent issuance of a global pharma quality manual, the company is standardising its quality controls for the industry worldwide.
- "By leveraging the know-how gained from adapting to the unique Japanese focus on quality and high affinity with GDP guidelines, as well as our experience in the United States, we hope to help the industry build a patient-centred pharmaceutical supply chain network on a global scale," says Otsuji.
*This content was paid for and produced by Nippon Express in partnership with the Commercial Department of the Financial Times.
